Quantum dynamics of hydrogen bonds
01/04/08 12:00 Filed in: single molecules
Water molecules are bound each other by hydrogen bond. In liquid water, three-dimensional networks of water moledules, formed by hydrogen bond, are rapidly changing their structure by incessant annihiration and re-creation of hydrogen bonds. It is believed that the chemical reaction in aqueous solution including those in vivo are influenced by the hydrogen bond.
Kumagai and Okuyama in our group succeeded in observing in real space the dynamics of hydrogen-bond alteration in water-dimer clusters isolated on a metal surface. They even revealed how the hydrogen bond alteration is governed by quantum mechanical tunneling effect. (Phys. Rev. Lett. 100, 166101 (2008))
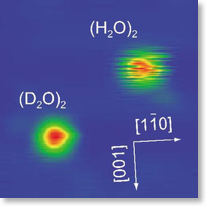 In this work, isolated water-dimer clusters were prepared on a Cu surface held at very low temperature, and its temporal behaviour was studied by scanning tunneling microscopy. The cluster is bonded to the surface via an oxygen atom in one of the two molecules. Scanning tunneling microscopic images show that the cluster is switching between two stable structures by altering the hydrogen bond. A subtle but important point in this remarkable observation is a large isotopic effect, which unambiguously imply that the quantum mechanical tunneling governs the switching process, which was further supported by a first-principles theoretical calculation.
In this work, isolated water-dimer clusters were prepared on a Cu surface held at very low temperature, and its temporal behaviour was studied by scanning tunneling microscopy. The cluster is bonded to the surface via an oxygen atom in one of the two molecules. Scanning tunneling microscopic images show that the cluster is switching between two stable structures by altering the hydrogen bond. A subtle but important point in this remarkable observation is a large isotopic effect, which unambiguously imply that the quantum mechanical tunneling governs the switching process, which was further supported by a first-principles theoretical calculation.