1. Structural Neuroscience
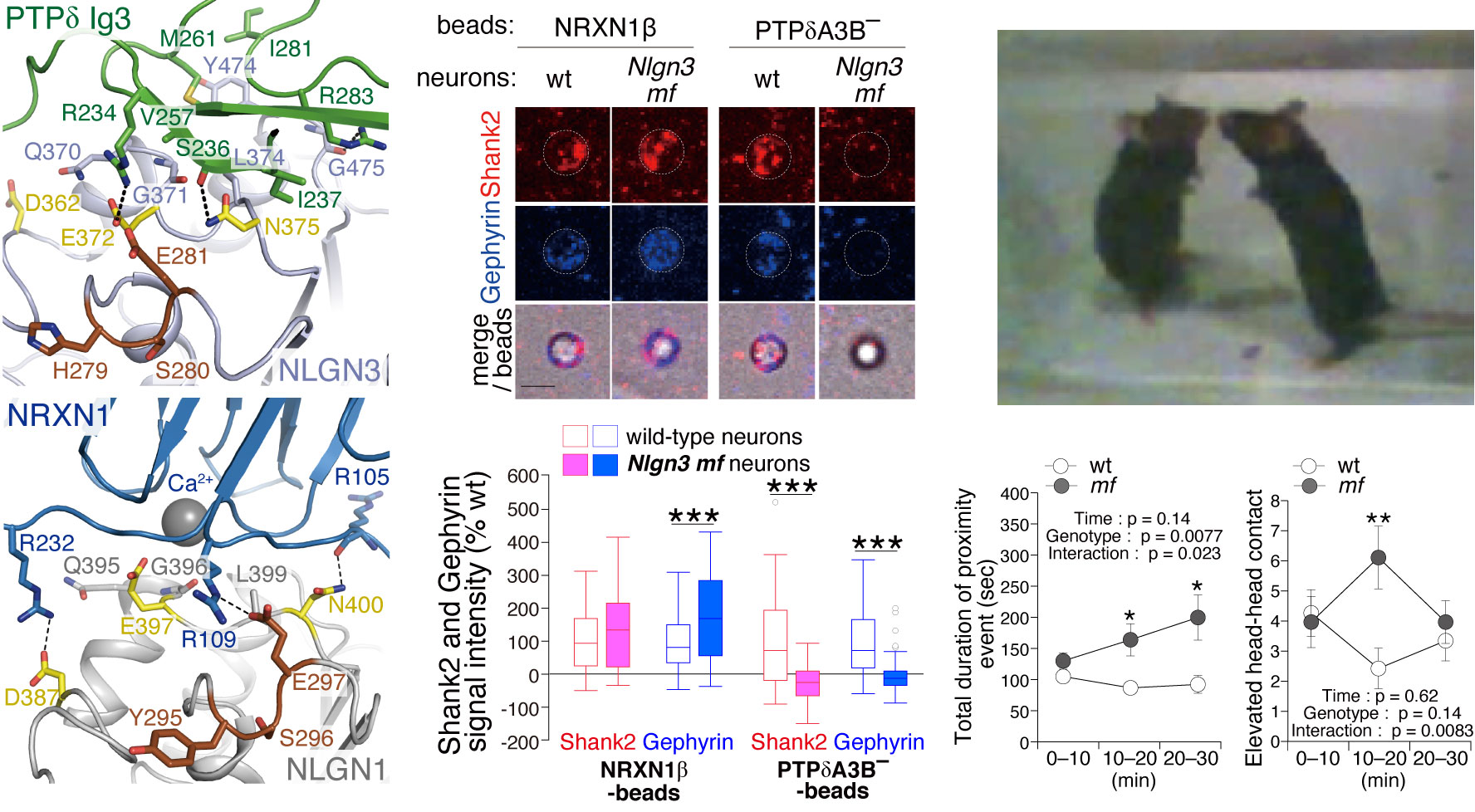
Synapse formation, a fundamental process in neurodevelopment, is triggered by interactions of cell adhesion molecules termed synapse organizers. Type IIa receptor protein tyrosine phosphatases (IIa RPTPs, also known as LAR-RPTPs) not only play important roles in axon guidance and neurite extension but also serve as presynaptic organizers. Transsynaptic interactions between IIa RPTPs and their partner postsynaptic organizers stimulate the accumulation of pre- and postsynaptic proteins in order to form subcellular structures specialized for the release of and response to neurotransmitters, respectively. Among three members of the IIa RPTP family, we focus on PTP𝜹. By X-ray crystallography and structure-based mutagenic analyses in vitro and in celluro, we have elucidated the mechanism underlying splice insert-specific pairing between PTP𝜹 and their cognate partners such as IL-1RAcPs, Slitrks, SALMs, and Neuroligin3 (NLGN3). In the latest study, we performed structure-based design of a NLGN3 mutation that selectively impairs either the non-canonical PTP𝜹-NLGN3 pathway or the canonical Neurexin-NLGN pathway and generated mice carrying such mutations. Behavior, cell biological, and electrophysical analyses of the mice suggested that the canonical and non-canonical NLGN3 pathways compete and regulate the development of sociality. An imbalance in these pathways may predispose to autism. Our findings provide novel mechanistic insights into the function of IIa RPTPs and reveal the structural basis for synapse formation induced by IIa RPTPs, which might support the future development of therapies for neurodevelopmental disorders.
Synapse Organizers
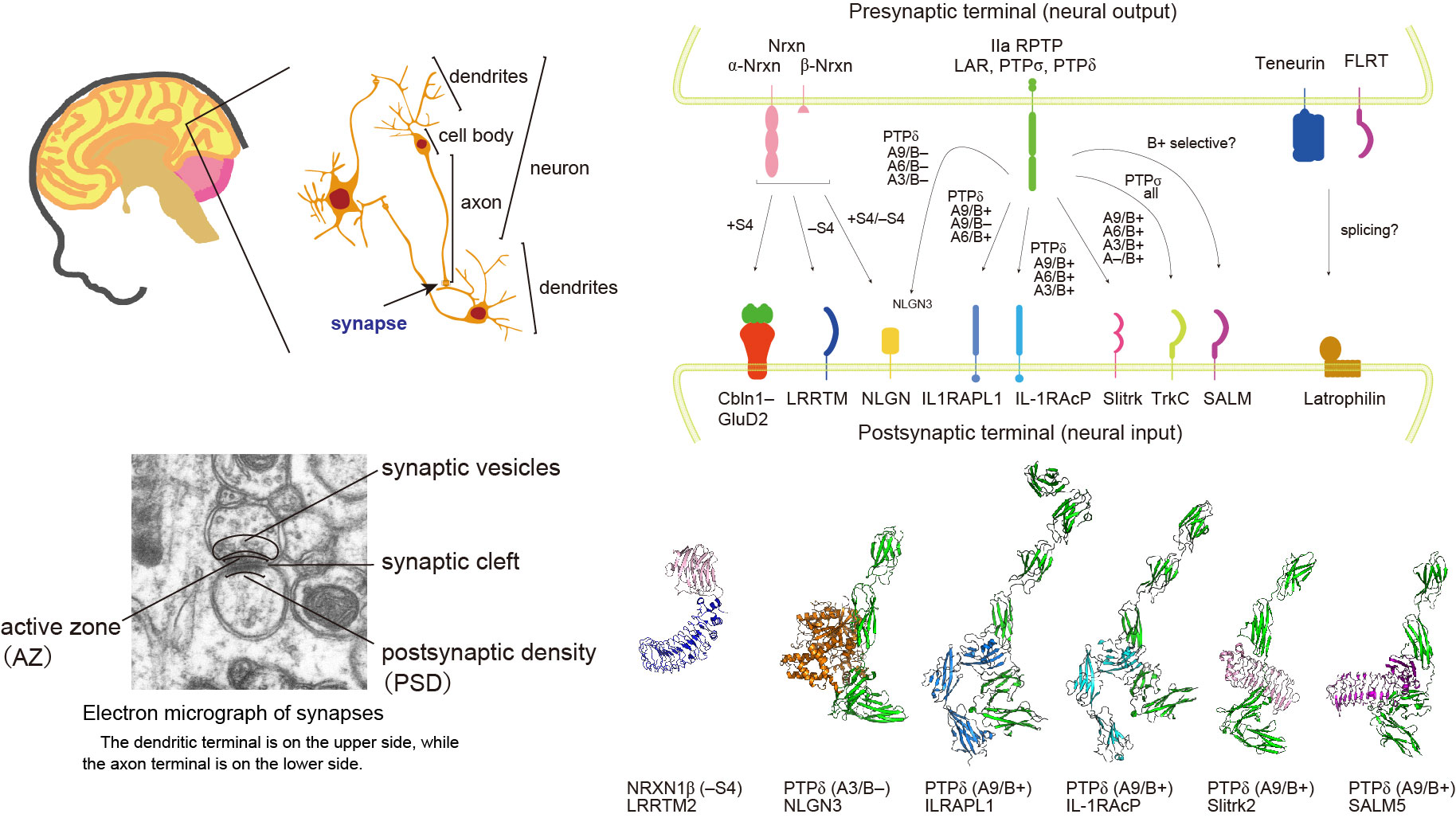
Understanding the Mechanism Underlying Synaptic Connections at the Atomic, Molecular, Cellular, and Behavioral Levels
2. Ubiquitin Signaling
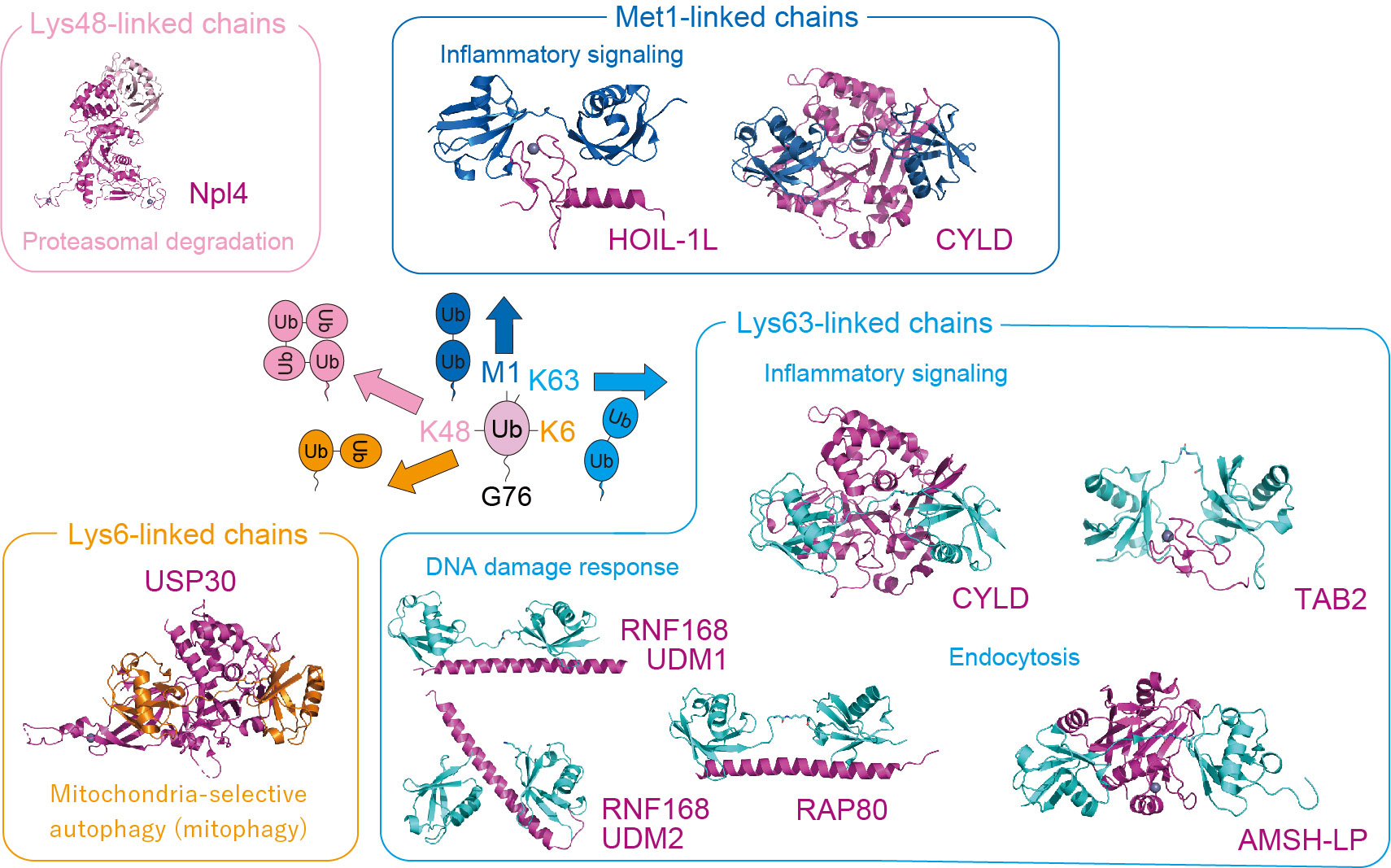
Ubiquitin is a small protein consisting of 76 amino acid residues and serves as a posttranslational modifier. Ubiquitin is well known for its function as a protein degradation signal by the proteasome. Meanwhile, recent research has revealed that ubiquitin also functions as a signaling molecule in various cellular contexts besides proteasomal degradation. Ubiquitin can polymerize to form a ubiquitin chain by being linked via isopeptide or peptide bonds between the amino group of lysine residues or the N-terminal methionine residue (M1) of one ubiquitin molecule and the carboxy group of the C-terminal glycine residue (G76) of another ubiquitin molecule. The ubiquitin chain linked via the 48th lysine (K48) acts as a degradation signal by the proteasome, whereas those linked via M1, K6, and K63 serve as proteasome-independent signals. We focus on DNA damage responses and inflammatory signals in which the M1 and K63 chains play important roles, and the mitochondria quality control, which is associated with the K6 chains and phosphorylated ubiquitin. We have revealed the signal control mechanism for these cellular processes through three-dimensional structural analysis and functional analysis. DNA damage and inflammation are closely related to cancer, whereas mitochondria quality control is to Parkinson's disease. We provide a structural platform for drug development.
The Ubiquitin Code
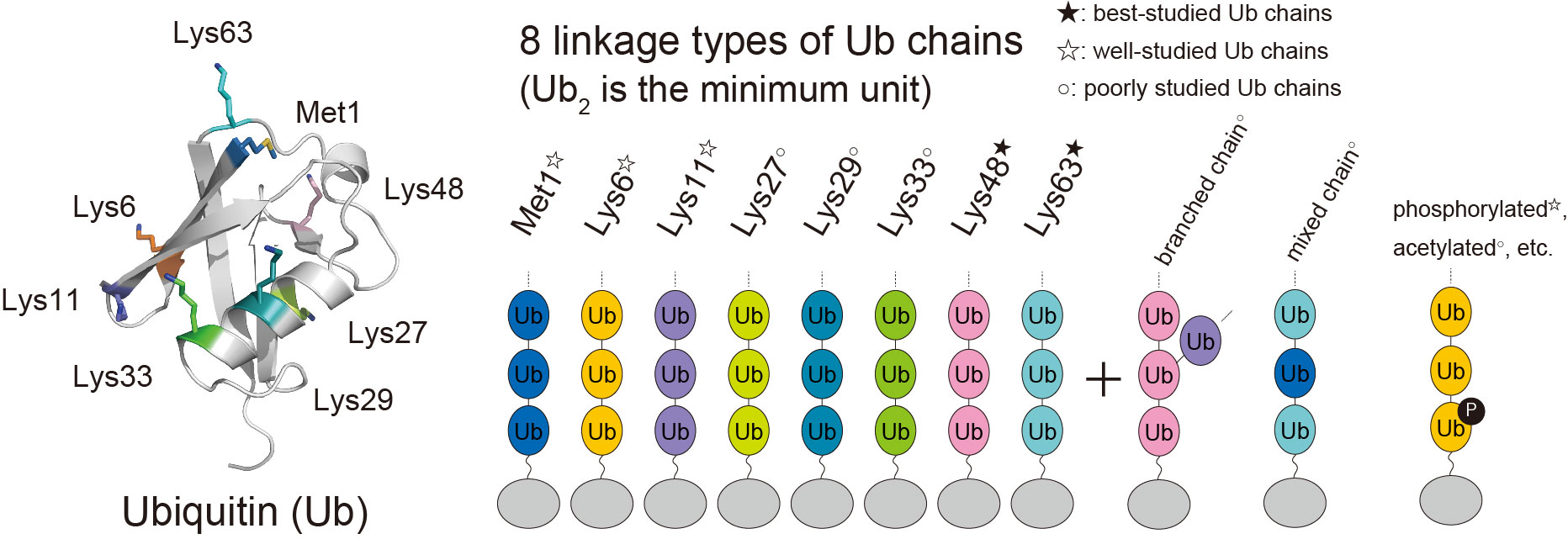
- ・Lys48-linked chains: proteasomal degradation
- ・Lys63-linked chains: endocytosis, inflammatory signaling, DNA damage response
- ・Met1-linked chains: inflammatory signaling
- ・Lys6-linked chains: mitochondria-selective autophagy (i.e., mitophagy)
- etc.
Customizing the Cellular Function by Manipulating the Molecular Signaling
3. Ultrahigh Resolution Analysis
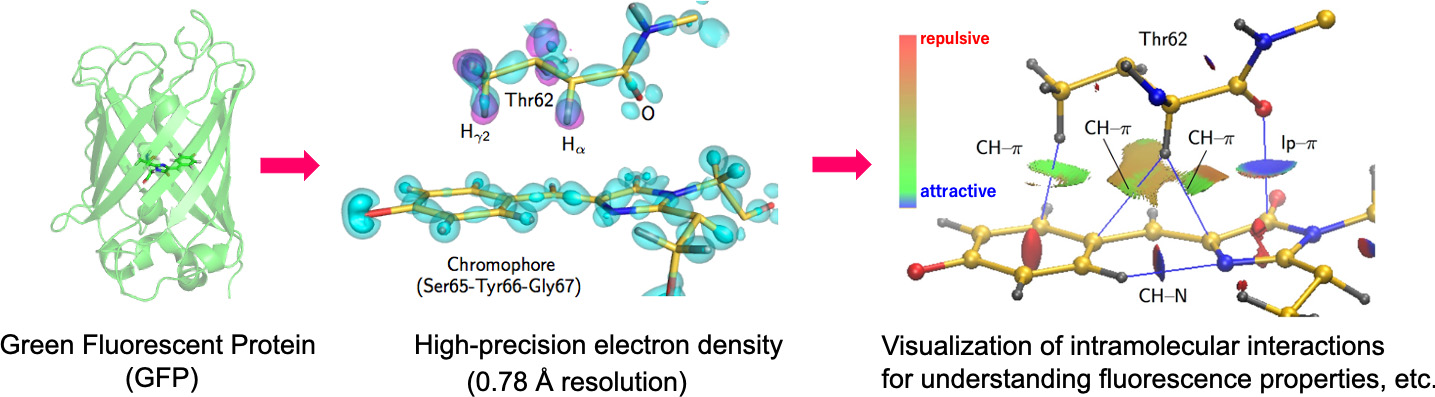
Ultrahigh resolution analysis exceeding 1 Å can capture subtle changes in electronic states and enables a precise chemical understanding of energy transmission and electron transfer in the biological system. We develop the methods of sample preparation, crystallization, measurement, and analysis for ultrahigh resolution analysis.
Experimentally Solving Electronic Structures Specifically Formed Inside Proteins and Understanding Their Functional Roles in the Reaction