
ResearchResearch
1.A.6 cryptochrome
Cryptochromes (CRY) are flavoproteins found in all kingdoms of life: plants
animals, and bacteria. CRYs share amino acid sequence similarities, a common
structural fold and the non-covalently bound flavin adenine dinucleotide
(FAD) cofactor with DNA photolyase (PHR) enzymes, which catalyze the light-dependent
repair of UV-damaged DNA. CRYs have little or no DNA repair activity, but
instead control a wide variety of life activities; light-dependent growth
and development in plants, the circadian clock in animals, and sensitivity
to the magnetic field (light-dependent magnetoreceptor). Among plant CRYs,
Arabidopsis thaliana Cryptochrome 1 (AtCRY1) has been most extensively
studied, while recent publications expand the fundamental knowledge about
the others such as Arabidopsis thaliana Cryptochrome 2 (AtCRY2) or algal
Cryptochrome. AtCRY1 regulates many aspects of photomorphogenesis. Plant
CRYs, represented by 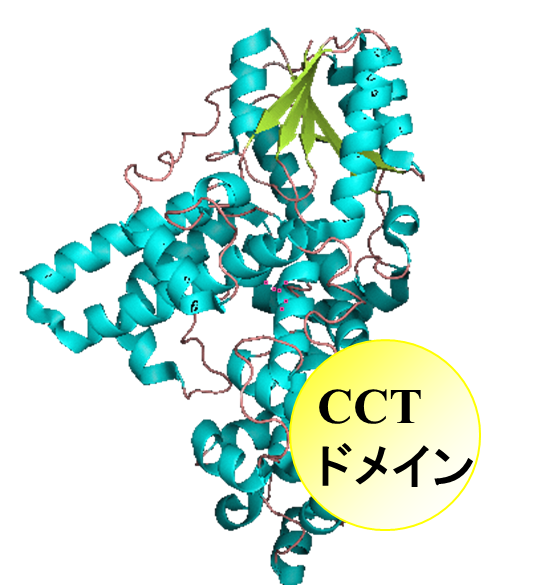 AtCRY1, contain not only the N-terminal PHR-like domain (c.a. 500 amino
acids), but also an additional characteristic Cryptochrome C-terminal (CCT)
domain. For plant CRYs, the CCT domain plays an important functional role;
genetic and physiological analysis of truncated forms of CRYs has demonstrated
that the CCT domain mediates signal transduction by interaction with protein
partners and can function in isolation when fused to a reporter gene. These
observations have led to the suggestion that CRY is activated through a
light-dependent conformational change that allows interaction with signaling
substrate. A similar mechanism has been proposed for drosophila CRY, except
that in this case signaling partner is thought to bind to the N-terminal
domain and the role of the C-terminus is to block access in a light dependent
manner through conformational change.
AtCRY1, contain not only the N-terminal PHR-like domain (c.a. 500 amino
acids), but also an additional characteristic Cryptochrome C-terminal (CCT)
domain. For plant CRYs, the CCT domain plays an important functional role;
genetic and physiological analysis of truncated forms of CRYs has demonstrated
that the CCT domain mediates signal transduction by interaction with protein
partners and can function in isolation when fused to a reporter gene. These
observations have led to the suggestion that CRY is activated through a
light-dependent conformational change that allows interaction with signaling
substrate. A similar mechanism has been proposed for drosophila CRY, except
that in this case signaling partner is thought to bind to the N-terminal
domain and the role of the C-terminus is to block access in a light dependent
manner through conformational change.
A significant reduction in the diffusion coefficient of AtCRY1 was observed upon photoexcitation, indicating that a large conformational change occurs in this monomeric protein. AtCRY1 containing a single mutation (W324F) that abolishes an intra-protein electron transfer cascade did not exhibit this conformational change. Moreover, the conformational change was much reduced in protein lacking the CCT domain. Thus, we conclude that the observed large conformational changes triggered by light excitation of the photolyase-like domain result from C-terminal domain rearrangement. This inter-domain modulation would be critical for CRYs’ abilility to transduce a blue-light signal into altered protein-protein interactions for biological activity.
(Back)


photo-physical-chemistry lab,京都大学大学院理学研究科 化学専攻 光物理化学研究室
〒606-8502
Kitashirakawaoiwakecho
Sakyoku, Kyoto, Japan
TEL +81-75-753-4026
FAX +81-75-753-4000
<Links for members>
Bake Web mail (Set up)
Manuals