
ResearchResearch
1.E.1 Cyt c
Molecular diffusion process after the photo-induced electron injection
to ferric cytorome c (Fe(III) cyt c) in guanidine hydrochloride (GdnHCl)
3.5 M buffer solution is studied by the time-resolved transient grating
technique. CD studies have revealed that Fe(III) cyt c is unfolded under
this condition but the reduced form, Fe(II) cyt c, is folded. Hence, this
pulsed laser induced reduction should initiate the folding process of cyt
c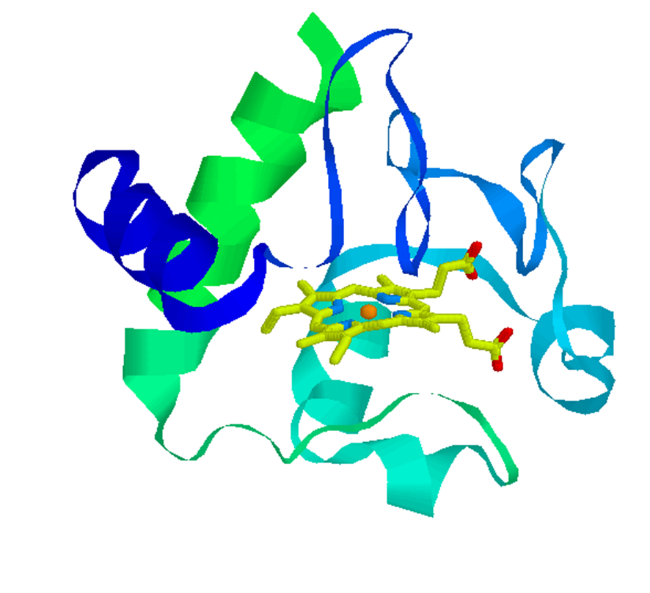 . The observed TG signal shows prominent features, which have never been
observed before. Based on several characteristic points, we concluded that
the apparent diffusion coefficient (D) of Fe(II) cyt c after the reduction
is time dependent, which must be associated with the protein folding dynamics.
This time dependent apparent D should reflect either the continuous time
development of the hydrodynamic radius or population change of the unfolded
and folded states during the folding dynamics. This is the first observation
of the time-dependent apparent D during any chemical reaction and this
time-dependent measurement of D should be a unique and powerful way to
study the protein folding kinetics from a view point of the proteins shape
or the protein-water intermolecular interaction.
. The observed TG signal shows prominent features, which have never been
observed before. Based on several characteristic points, we concluded that
the apparent diffusion coefficient (D) of Fe(II) cyt c after the reduction
is time dependent, which must be associated with the protein folding dynamics.
This time dependent apparent D should reflect either the continuous time
development of the hydrodynamic radius or population change of the unfolded
and folded states during the folding dynamics. This is the first observation
of the time-dependent apparent D during any chemical reaction and this
time-dependent measurement of D should be a unique and powerful way to
study the protein folding kinetics from a view point of the proteins shape
or the protein-water intermolecular interaction.
Change of the diffusion coefficient of Cyt c during the refolding process
was also traced in time domain from the unfolded value to the native value
continuously at various denaturant (guanidine hydrochloride (GdnHCl)) concentrations
and temperatures. In the temperature range of 288 K-308 K and GdnHCl concentration
range of 2.5 M- 4.25 M, the diffusion change can be analyzed well by the
two state model consistently. It was found that the m‡-value and the activation
energy of the transition state from the unfolded state for the hydrogen
bonding network change are surprisingly similar to that for the local structural
change around the heme group monitored by the fluorescence quenching experiment.
This agreement suggests the existence of common or similar fundamental
dynamics including water molecular movement to control the refolding dynamics.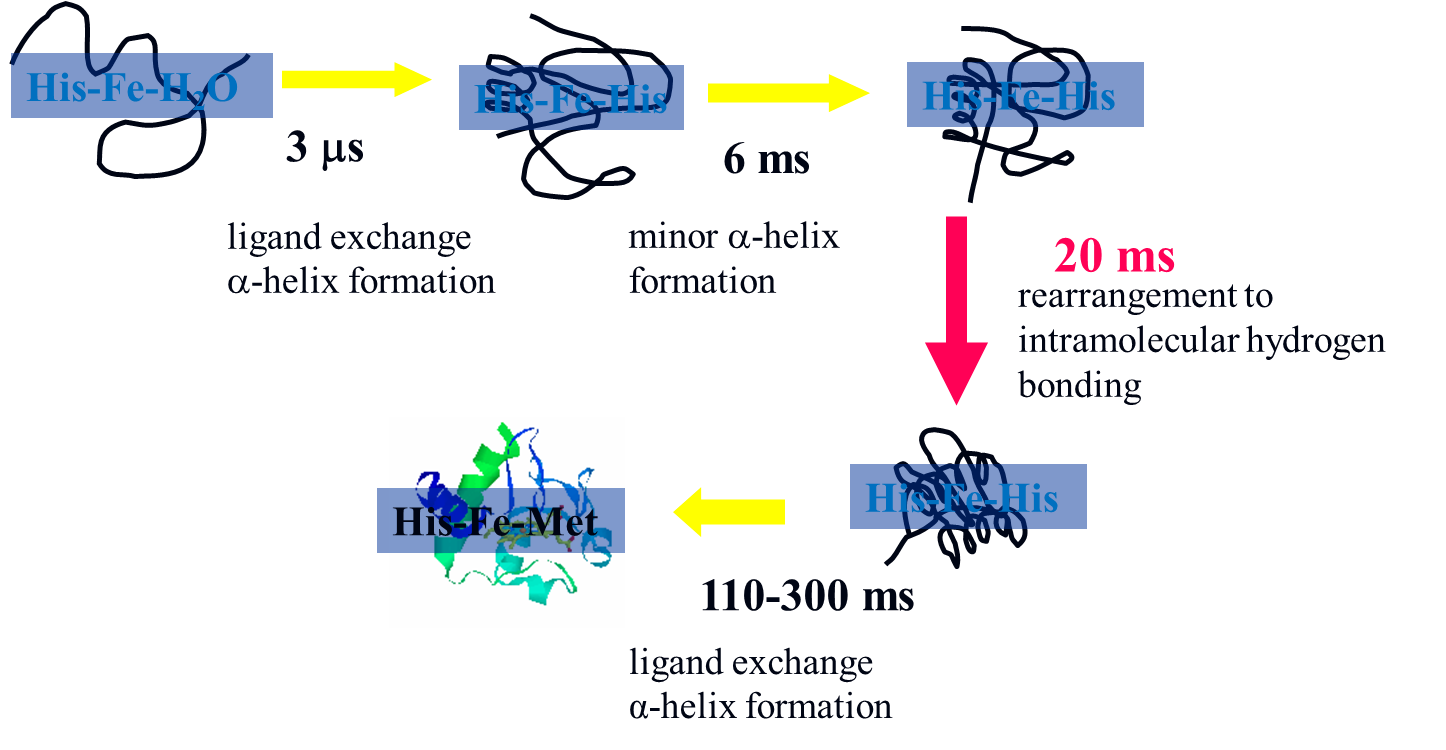
(Back)


photo-physical-chemistry lab,京都大学大学院理学研究科 化学専攻 光物理化学研究室
〒606-8502
Kitashirakawaoiwakecho
Sakyoku, Kyoto, Japan
TEL +81-75-753-4026
FAX +81-75-753-4000
<Links for members>
Bake Web mail (Set up)
Manuals