
ResearchResearch
6. Energy Relaxation and Photothermal Process in Solution
In the field of chemistry, we are very much interested in dynamics of
molecules with hoping that we can ultimately understand chemical reactions
completely; the 'dynamics' include dynamical processes of molecules in
space as well as energy transfer processes among various intramolecular
states or intermolecular systems. The energy flow process from solute to
solvent is of major importance not only in chemistry but also in other
fields of science such as laser ablation in industrial technology or biology,
and has been a subject of extensive researches during the last two decades.
The excess energy due to the nonradiative transition between electronic
states (internal conversion and/or intersystem crossing) will be first
transferred to several energy-accepting vibrational modes, so that the
total energy is conserved before and after the transition. The energy ultimately
goes to the translational freedom (heating). This phenomenon is known as
the photothermal effect and is one of the most commonly observed phenomena
after the photoexcitation in condensed phase. Revealing the elementary
step of this energy relaxation process has been one of the central topics
in physical chemistry. The energy transfer process from the photoexcited
molecule to the matrix has been studied so far by monitoring the population
decay from highly excited vibrational states by the time-resolved Raman
scattering, transient IR spectroscopy the hot band detection of the solvent
or solute molecules, or the stimulated emission detection. A number of
these results so far show that most of the vibrational relaxations of polyatomic
molecules in solution are in a range of 100 ps - 10 ps. The temperature
of the matrix rises because of the released energy from the highly excited
vibrational states. Hence, it is reasonable to consider that the translational
temperature rise also in a range of 100 ps-10 ps even if the equilibrium
process in the translational freedom is extremely fast. However, by using
the direct temperature detection, we showed that this consideration is
not correct.
Compared with these rather extensive investigations of the vibrational
cooling processes, studies of the heating process of the matrix, i.e.,
increase of the thermal energy, which is the ultimate energy-accepting
mode in the condensed phase, are very rare. So far, several photothermal
detection methods have been developed for measuring the temperature increase.
However, although improvement on the sensitivity has been pursued extensively
for a long time, it is not so much with respect to the time resolution.
This inherent time response comes from the fact that most of the photothermal
detection methods are using a matrix density change by the temperature
increase as the source of the signal. In an effort to understand the thermalization
from a point of view of the translational energy of the solvents, we developed
four new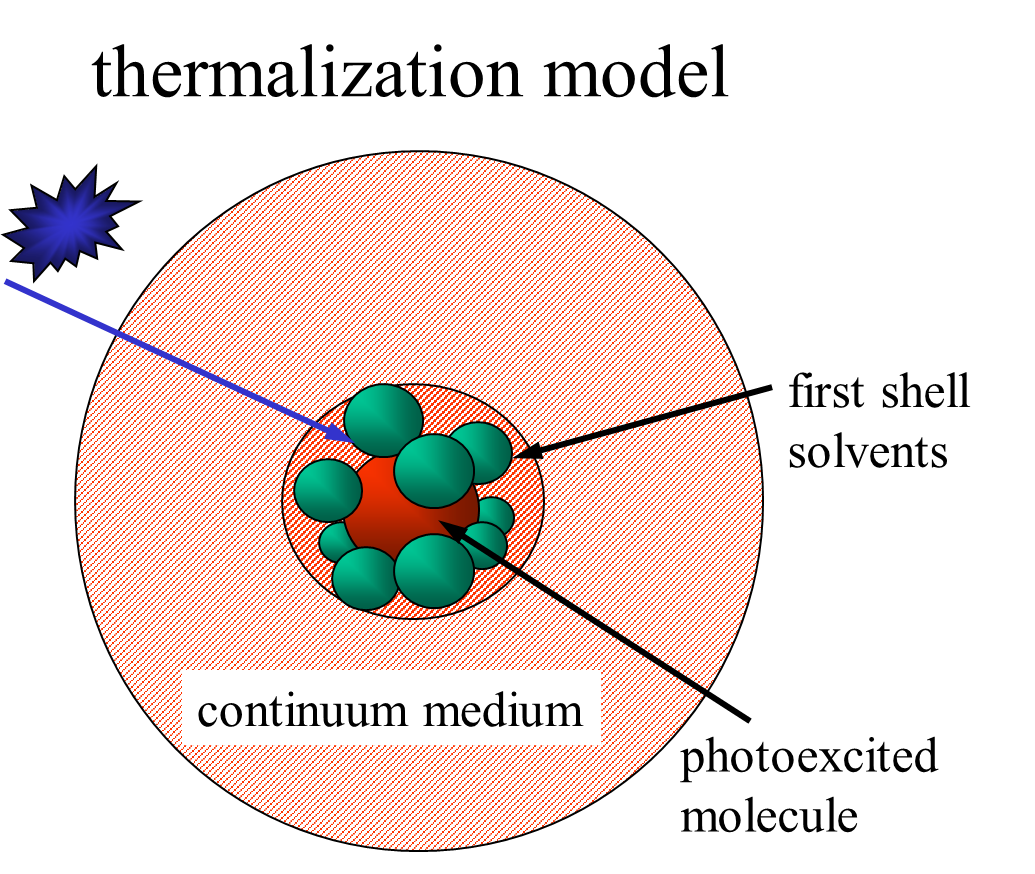 methods: temperature lens, temperature grating, an acoustic peak shift
method and molecular heater-molecular thermometer system. Our results showed
that the temperature rise after the decay of the electronic state is quite
fast in water. When the solvent motions are coupled strongly with the large
energy fluctuation, that fluctuation may efficiently transfer the internal
energy to the kinetic energy. Hence it is expected that the energy transfer
depends on the nature of the solute-solvent interaction as well as that
of the solvent-solvent interaction. The mechanism and the rate of the temperature
rise were further explored in various systems by changing the matrices
using the acoustic peak shift method. A new molecular integrated system:
molecular heater-molecular thermometer, is described as a new trial for
the study of the temporal and spatial propagation of the thermal energy
from the "hot" molecules.
methods: temperature lens, temperature grating, an acoustic peak shift
method and molecular heater-molecular thermometer system. Our results showed
that the temperature rise after the decay of the electronic state is quite
fast in water. When the solvent motions are coupled strongly with the large
energy fluctuation, that fluctuation may efficiently transfer the internal
energy to the kinetic energy. Hence it is expected that the energy transfer
depends on the nature of the solute-solvent interaction as well as that
of the solvent-solvent interaction. The mechanism and the rate of the temperature
rise were further explored in various systems by changing the matrices
using the acoustic peak shift method. A new molecular integrated system:
molecular heater-molecular thermometer, is described as a new trial for
the study of the temporal and spatial propagation of the thermal energy
from the "hot" molecules.
(Back)


photo-physical-chemistry lab,京都大学大学院理学研究科 化学専攻 光物理化学研究室
〒606-8502
Kitashirakawaoiwakecho
Sakyoku, Kyoto, Japan
TEL +81-75-753-4026
FAX +81-75-753-4000
<Links for members>
Bake Web mail (Set up)
Manuals