
ResearchResearch
1.F Photo-dissociation reaction of carboxymyoglobin
The relationship between thestructural properties of proteins and their
reactions is essentially importantfor understanding the protein biological
functions. Myoglobin has been used as a model system for experimental and
theoretical studies of such a kinetics-structural relation. A heme is embedded
within the protein and a small ligand (e.g., O2, CO, NO) is reversibly
bind tot he sixth coordination site, on the distal side, of the heme. By
the photoexcitation of the heme, the ligand-metal bond is photodissociated.
Since there is no route prepared for the ligand to pass through the protein
to the outer solvent, the protein structure has to change if the ligand
is removed from the protein. This photoreaction can be used, therefore,
to trigger a perturbation to the protein by pulsed laser light. Since most
of kinetic studies have been conducted by optical spectroscopies, which
use the optical transition of the heme, they probed only the local structural
change around the heme but give little information about the global structural
change of the molecule such as protein motion. Therefore, there are many
unresolved questions in the dissociation-association process of MbCO in
spite of a large number of studies. For example, although the 180 ns-dynamics
at 20 °C was sometimes regarded as the CO escaping process from the protein
to solvent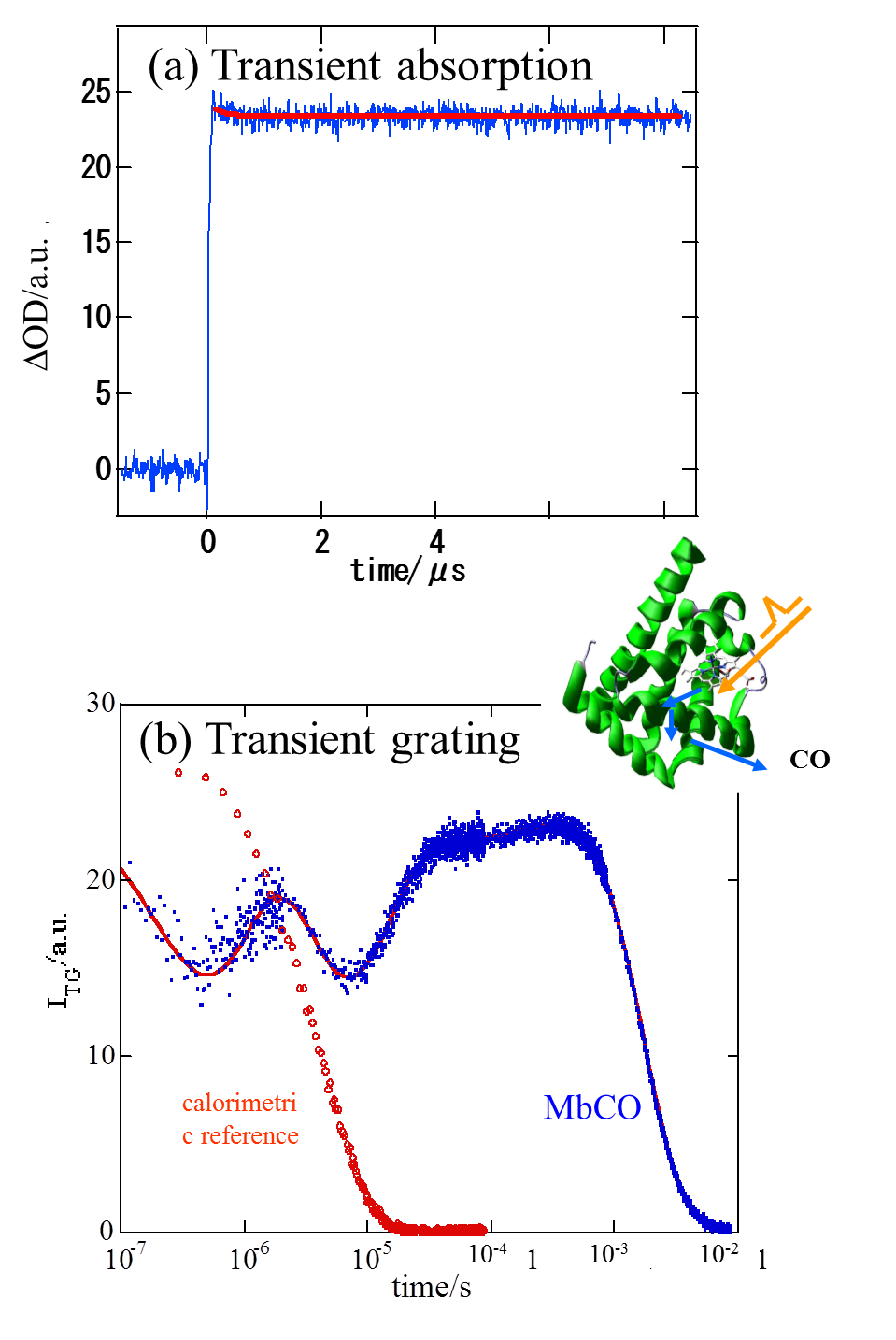 , it could be just CO moving inside the protein. How fast does the CO escape
from the protein matrix to the solvent? Relating with this question, we
might have another one; how many intermediates are there in the time course
from the photodissociation to the CO escape? If there are several intermediates,
how much energy do the intermediates have? The energies of the intermediates
are very important information, but our knowledge of this problem is very
limited even after the long history of the Mb studies. These difficulties
may be solved by using a time-resolved spectroscopy that does not use the
optical transition of the heme. We performed a quantitative investigation
on the dynamics of the protein of horse heart carboxymyoglobin (MbCO) after
photodissociation of CO from 10 ns to milliseconds continuously by a new
technique; the laser induced transient grating (TG) and photoacoustic (PA)
hybrid method. We found that initially, ~10 ns after thephotodissociation,
a small volume contraction takes place (5 ml/mol) and an energy of 61 kJ/mol
is stored in the system. This energy is smaller than the Fe-CO bond energy.
This fact indicates a rather large protein structural relaxation in this
fast time scale. Subsequently a structural change, volume expansion of
14.7 ml/mol, occurs with a lifetime of 700 ns at 20 C.This indicates the
presence of the second intermediate state, from where the ligand cannot
return back to the heme, and the 700 ns process is attributed to the escaping
of CO from the protein to the outer solvent phase. Interestingly we found
that this volume change is actually depend on the temperature. The energies
and the structural relaxation of the protein are discussed.
, it could be just CO moving inside the protein. How fast does the CO escape
from the protein matrix to the solvent? Relating with this question, we
might have another one; how many intermediates are there in the time course
from the photodissociation to the CO escape? If there are several intermediates,
how much energy do the intermediates have? The energies of the intermediates
are very important information, but our knowledge of this problem is very
limited even after the long history of the Mb studies. These difficulties
may be solved by using a time-resolved spectroscopy that does not use the
optical transition of the heme. We performed a quantitative investigation
on the dynamics of the protein of horse heart carboxymyoglobin (MbCO) after
photodissociation of CO from 10 ns to milliseconds continuously by a new
technique; the laser induced transient grating (TG) and photoacoustic (PA)
hybrid method. We found that initially, ~10 ns after thephotodissociation,
a small volume contraction takes place (5 ml/mol) and an energy of 61 kJ/mol
is stored in the system. This energy is smaller than the Fe-CO bond energy.
This fact indicates a rather large protein structural relaxation in this
fast time scale. Subsequently a structural change, volume expansion of
14.7 ml/mol, occurs with a lifetime of 700 ns at 20 C.This indicates the
presence of the second intermediate state, from where the ligand cannot
return back to the heme, and the 700 ns process is attributed to the escaping
of CO from the protein to the outer solvent phase. Interestingly we found
that this volume change is actually depend on the temperature. The energies
and the structural relaxation of the protein are discussed.
We also investigated the photodissociation kinetics of sperm whale (SW)
MbCO by the TG and PA hybrid method. SW-Mb is the most frequently studied
Mb, and the dynamics as well as the enthalpy change and volume change of
this protein are important information. Besides this, there are mainly
three purposes. We developed a new method of the TG analysis of the CO
diffusion process under various grating wavenumbers to determine the rate
of the ligand escape from the protein interior. Using this analysis, we
can definitively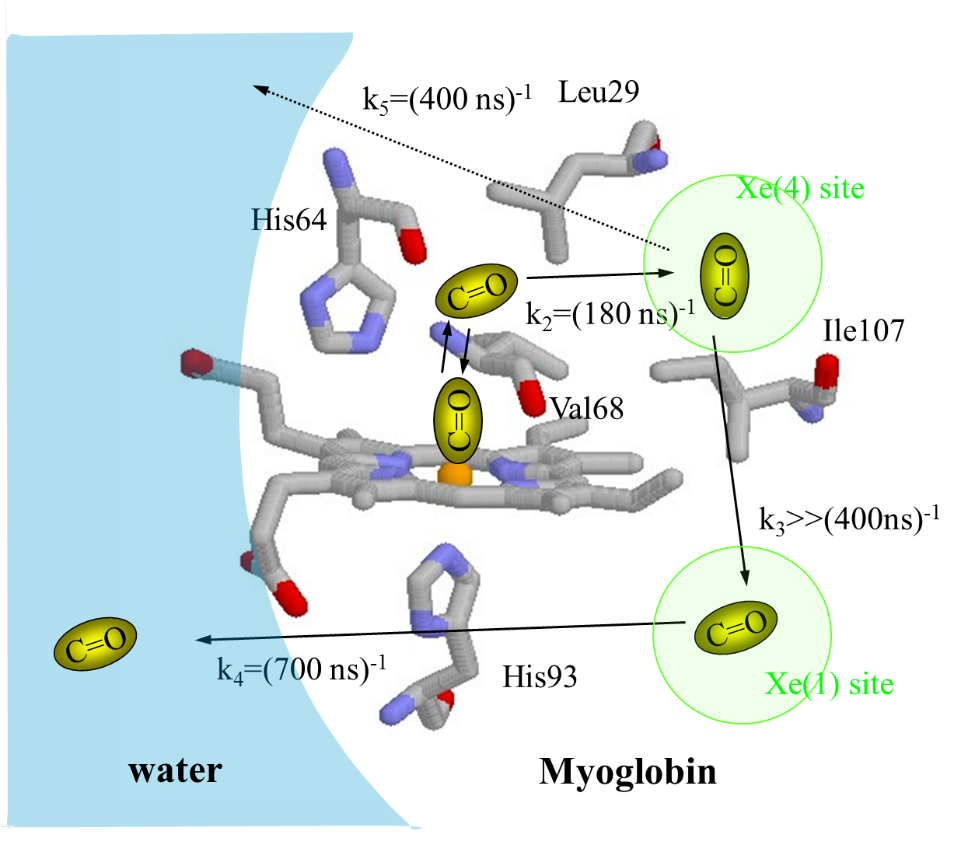 show that the 700 ns-dynamics represents the CO escaping process. From
these results, we conclude that there are at least two intermediate states
during the course of the CO dissociation. Hence, a four-state model is
appropriate to describe the ligand dissociation reaction even at room temperature.
The dynamics of SW-MbCO is compared with that of HH-MbCO. There are 21
amino acid residues that are different between HH- and SW-Mb. Comparing
these two proteins, we can examine how the observed dynamics is sensitive
to these amino acid residues. In particular, the comparison will give us
a clue on the role of salt bridge between Arg45 and heme 6-propionate side
chain. We examined the participation of the salt bridge dynamics in the
CO dissociation reaction.
show that the 700 ns-dynamics represents the CO escaping process. From
these results, we conclude that there are at least two intermediate states
during the course of the CO dissociation. Hence, a four-state model is
appropriate to describe the ligand dissociation reaction even at room temperature.
The dynamics of SW-MbCO is compared with that of HH-MbCO. There are 21
amino acid residues that are different between HH- and SW-Mb. Comparing
these two proteins, we can examine how the observed dynamics is sensitive
to these amino acid residues. In particular, the comparison will give us
a clue on the role of salt bridge between Arg45 and heme 6-propionate side
chain. We examined the participation of the salt bridge dynamics in the
CO dissociation reaction.
Protein dynamics observed by the transient grating (TG) method were studied
for some site-directed mutants at the distal histidine of myoglobin (H64L,
H64Q, H64V). The time profiles of the TG signals were very sensitive to
the amino acid residue of the 64 position. It was found that the sensitivity
is mostly caused by the different rates of the ligand escape from the protein
to solvent and the magnitude of the molecular volume change. Several molecular
origins of the volume difference between MbCO and Mb were proposed. Interestingly,
the volume difference between the CO-trapped Mb inside the protein interior
and Mb is similar to that of the partial molar volume of CO in solvent.
Nature of the CO trapped cite was discussed.
(Back)


photo-physical-chemistry lab,京都大学大学院理学研究科 化学専攻 光物理化学研究室
〒606-8502
Kitashirakawaoiwakecho
Sakyoku, Kyoto, Japan
TEL +81-75-753-4026
FAX +81-75-753-4000
<Links for members>
Bake Web mail (Set up)
Manuals