
ResearchResearch
1.A.5 (c) PixD
(i) SyPixD
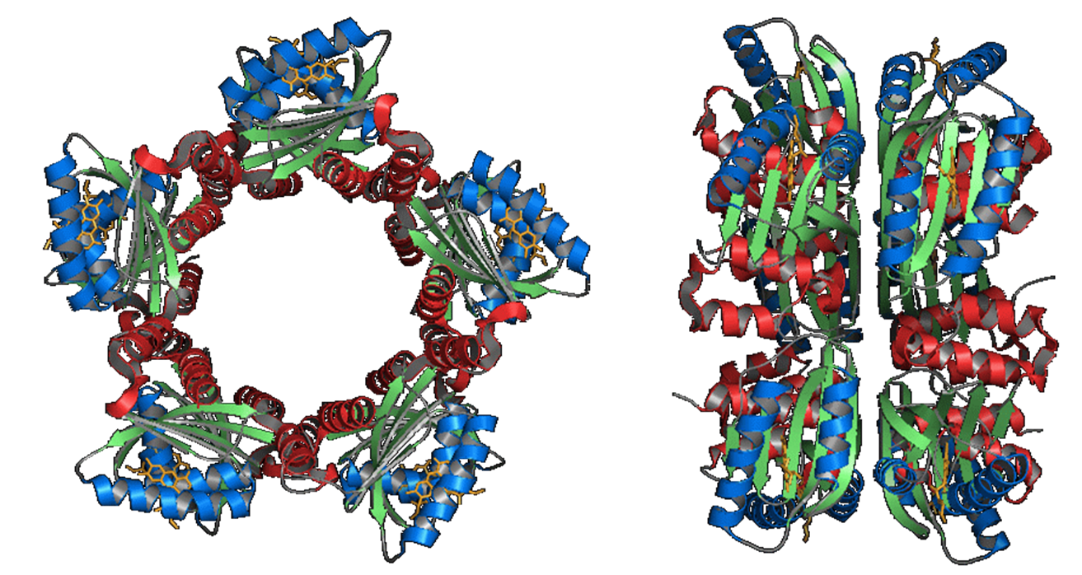
SyPixD is a BLUF protein from Synechocystis sp. PCC6803 (Slr1694). According
to the crystal structure, PixD consists of the BLUF domain and additional
helices. The most striking characteristic of PixD is the unique formation
of oligomers. PixD was found to crystallize to form a decamer in the asymmetric
unit with two pentameric rings. This decameric structure may play an important
role in the signal transduction of PixD proteins.
A conformational change coupled with a volume contraction of 13 mL mol−1
was observed with a time constant of 45 ms following photoexcitation. At
a weak excitation light intensity, there were no further changes in the
volume and the diffusion coefficient (D). The determined D-value (3.7 ×
10−11 m2 s−1) suggests that PixD exists as a decamer in solution, and this
oligomeric state was confirmed by size exclusion chromatography (SEC) and
blue native-polyacrylamide gel electrophoresis. Surprisingly, by increasing
the excitation laser power, a large increase in D with a time constant
of 350 ms was observed following the volume contraction reaction. The D-value
of this photoproduct species (7.5 × 10−11 m2 s−1) is close to that of the
PixD dimer. Combined with TG and SEC measurements under light-illuminated
conditions, the light-induced increase in D was attributed to a transient
dissociation reaction of the PixD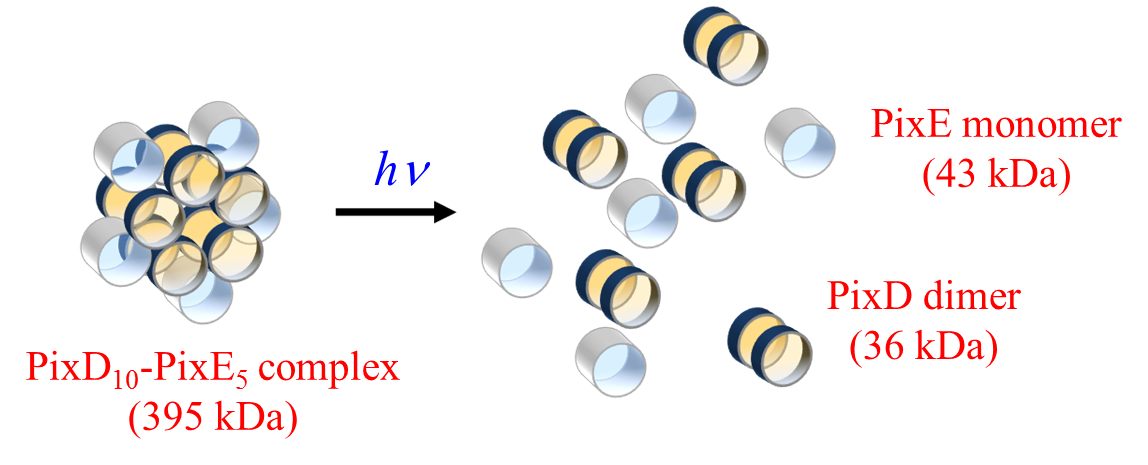 decamer to a dimer. For the M93A-mutated PixD, no volume or D-change was
observed. Furthermore, we showed that the M93A mutant did not form the
decamer but only the dimer in the dark state. These results indicate that
the formation of the decamer and the conformational change around the Met
residue are important factors that control regulation of the downstream
signal transduction by the PixD photoreceptor.
decamer to a dimer. For the M93A-mutated PixD, no volume or D-change was
observed. Furthermore, we showed that the M93A mutant did not form the
decamer but only the dimer in the dark state. These results indicate that
the formation of the decamer and the conformational change around the Met
residue are important factors that control regulation of the downstream
signal transduction by the PixD photoreceptor.
A protein-to-protein interaction between PixD and a response regulator
PixE (Slr1693) is essential to achieve light signal transduction for phototaxis
of the species. We studied interprotein reaction dynamics using time-resolved
transient grating spectroscopy. The dissociation process was clearly observed
as the light-induced diffusion coefficient change in the time domain and
the kinetics was determined. More strikingly, disassembly was found to
take place only after photoactivation of two PixD subunits in the complex.
This result suggests that the biological response of PixD does not follow
a linear correlation with the light intensity, but appears to be light-intensity-dependent.
(ii) TePixD
TePixD is a BLUF protein from Thermosynechococcus elongatus BP-1 (Tll0078).
After formation of an intermediate species with a red-shifted absorption
spectrum, two new reaction phases reflecting protein conformational changes
were discovered; one reaction phase manifested itself as the expansion
of the partial molar volume with a time constant of 40 microsec, whereas
the other reaction phase represented a change in the diffusion coefficient
(D) (i.e. the diffusion-sensitive conformational change (DSCC)). D decreased
from 4.9 × 10−11 to 4.4 × 10−11 m2 s−1 upon the formation of the first
intermediate, and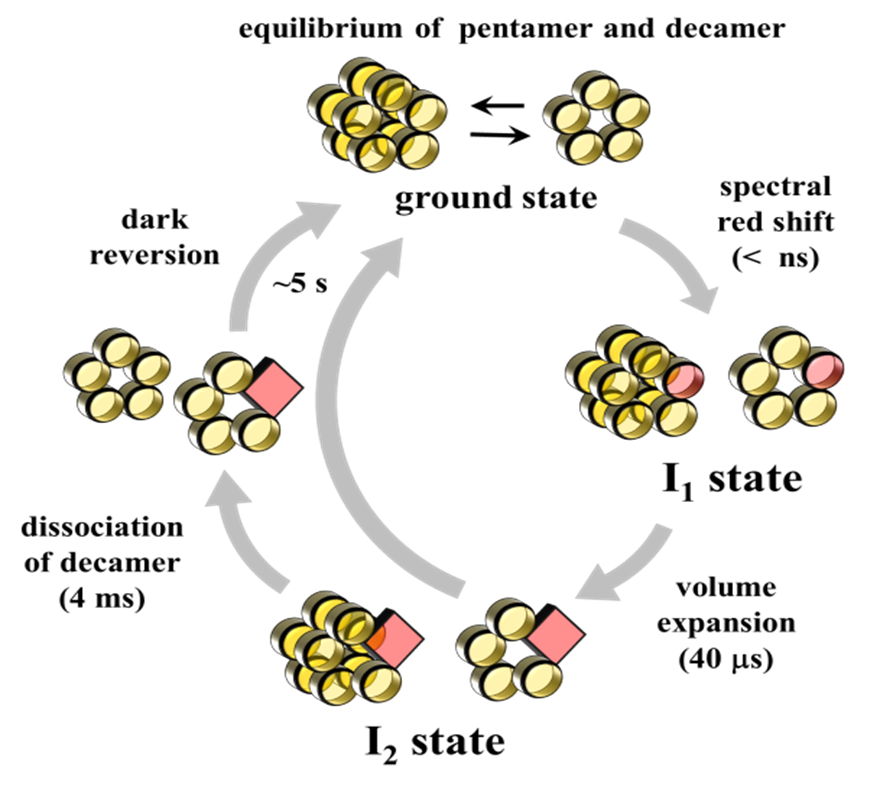 subsequently showed a more pronounced decrease to 3.2 × 10−11 m2 s−1 upon
formation of the second intermediate. From the global analysis of the signals
at various grating wavenumbers, the time constant of the D-change was determined
to be 4 ms. Although the magnitude and the rate constant of the faster
volume change was independent of protein concentration, the amplitude of
the signal which reflects the later DSCC significantly decreased as the
protein concentration decreased. This concentration dependence suggests
that two species exist in solution; a reactive species exhibiting the DSCC
and a second species which is non-reactive. The fraction of these species
was found to be dependent on the concentration. The difference in the reactivity
was attributed to the different oligomeric states of TePixD, i.e. pentamer
and decamer. The equilibrium of these states in the dark was confirmed
by size-exclusion chromatography at various concentrations. These results
demonstrated that only the decamer state is responsible for the conformational
change. The results may suggest that the oligomeric state is functionally
subsequently showed a more pronounced decrease to 3.2 × 10−11 m2 s−1 upon
formation of the second intermediate. From the global analysis of the signals
at various grating wavenumbers, the time constant of the D-change was determined
to be 4 ms. Although the magnitude and the rate constant of the faster
volume change was independent of protein concentration, the amplitude of
the signal which reflects the later DSCC significantly decreased as the
protein concentration decreased. This concentration dependence suggests
that two species exist in solution; a reactive species exhibiting the DSCC
and a second species which is non-reactive. The fraction of these species
was found to be dependent on the concentration. The difference in the reactivity
was attributed to the different oligomeric states of TePixD, i.e. pentamer
and decamer. The equilibrium of these states in the dark was confirmed
by size-exclusion chromatography at various concentrations. These results
demonstrated that only the decamer state is responsible for the conformational
change. The results may suggest that the oligomeric state is functionally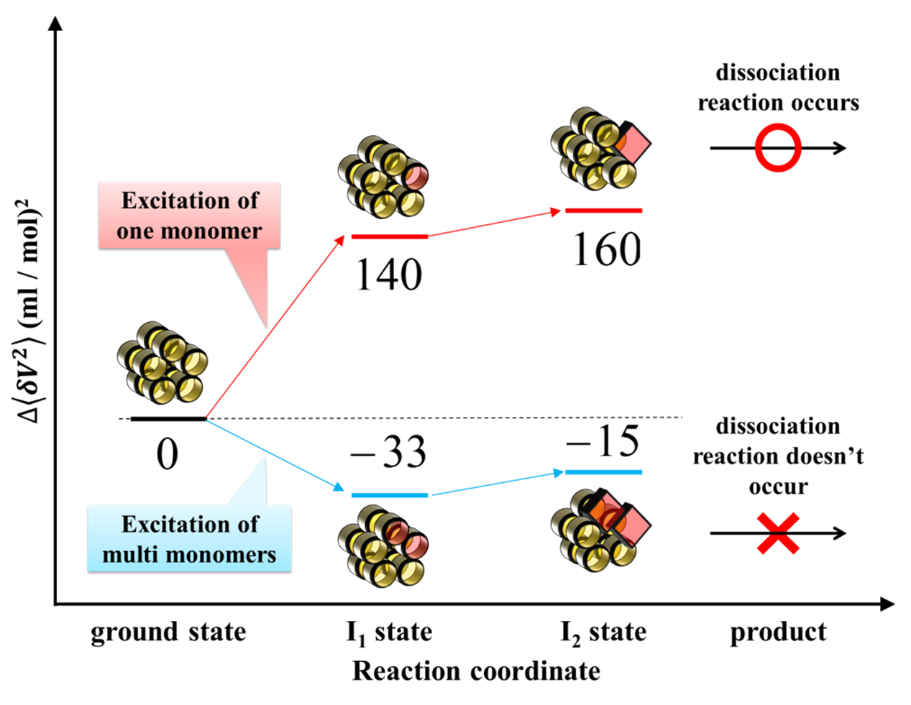 important in signal transduction of this photosensory protein.
important in signal transduction of this photosensory protein.
While the number of excited molecules increased monotonically as the
laser power increased, the number of decamers exhibiting a global conformational
change initially increased, and began to decrease with increasing the excitation
intensity. This unusual power dependence was analyzed based on a Poisson
distribution equation, demonstrating that decamers containing more than
one excited monomer subunit do not undergo conformational change. Our results
suggest that TePixD has a function of not only a photosensor but also sensing
the light intensity.
(Back)


photo-physical-chemistry lab,京都大学大学院理学研究科 化学専攻 光物理化学研究室
〒606-8502
Kitashirakawaoiwakecho
Sakyoku, Kyoto, Japan
TEL +81-75-753-4026
FAX +81-75-753-4000
<Links for members>
Bake Web mail (Set up)
Manuals